Atoms and Ions
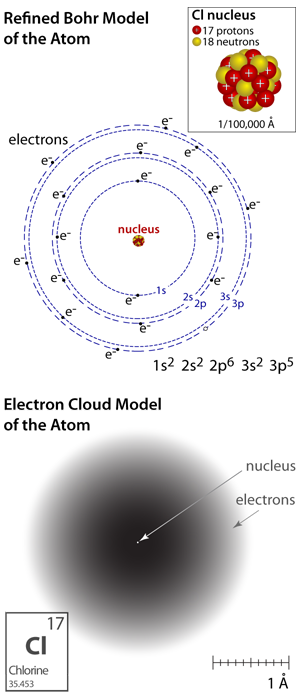
All matter is made of atoms! And atoms are made of even smaller more fundamental particles. Atoms are not shiny little rigid spheres as we depict in drawings and simulations; they're more like a squishy storm of tiny particles swirling about a dense nucleus. Almost all of the mass of an atom is actually contained within the nucleus, which is comprised of two different types of primary particles: the proton (a positively charged particle) and the neutron (which does not have a charge). Some distance away even smaller negatively charged particles called electrons are continuously orbiting the positively charged nucleus (e– is defined as the elementary negative charge). Atoms have the same number of electrons and protons and the charge of a proton is the same magnitude as the charge of an electron; thus, the net charge of an atom is zero.
Models of an atom: In the refined Bohr model of the atom the electrons are shown orbiting the nucleus in specific electron configurations, like planets orbit the sun along specific paths. In the electron cloud model of the atom, the shading is defined by the probability that an electron would be found in a specific location.
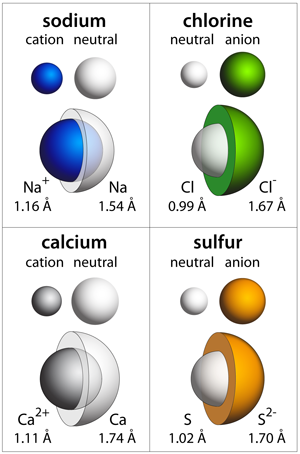
Ionic Radii: The crystal ionic radii as compared to the neutral atom radii — sodium, chlorine, calcium, and sulfur. The neutral atom radii are shown in white while the ionic radii are colored.
If an atom loses or gains an electron then the net charge of the atom is no longer zero, and this atom is now called an ion. The ionic charge is defined by the difference between the number of electrons and protons. Thus, there are two basic types of ions (positively charged ions and negatively charged ions). A positively charged ion is called a cation, and it has lost an electron — the amount of net positive charge depends on how many electrons have been removed (+1, +2, or +3). Conversely, a negatively charged ion (called an anion) has gained some number of electrons; anions will have typically have charges of –1, –2, or –3.
The radius of an ion will be larger or smaller than the neutral atom depending on whether the ion has gained or lost an electron, respectively. Cations, which have lost at least 1 electron, carry a positive charge and their ionic radius is smaller due to this charge imbalance. Anions, on the other hand, have gained one or more electrons and have a net negative charge; anions have a larger radius due to these excess electrons. The figure to the left shows the ionic radii (as deduced from crystallographic measurements of solids) as compared to the atomic radii for the neutral atoms.
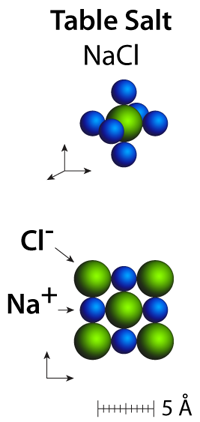
Crystal structures: The packing arrangements in ionic solids are functions of both the ionic charges and the ionic radii. In the case of sodium chloride, the ions pack into square patterns with 4 nearest neighbors of the opposite charge within a plane, and in three dimensions they pack into cubes where each ion has 6 neighbors of opposite charge.
Ionic Bonding
Because ions are charged, they follow the same set of rules that all charged particles obey: they are attracted to oppositely charged particles and are repelled by particles with a like charge. Thus, anions and cations are attracted to each other, but anion–anion and cation–cation interactions are always repulsive. Ionic bonding arises from these incredibly strong attraction forces.
Coulomb's law describes the force of attraction (F) between two charged particles, and is given by the formula below.
F = – C (z1 z2) / R2
In the above equation, the force (F) is positive (attractive) only if the ions have opposite charges (z1 and z2 are the charges of the ions). The force is also proportional to one over the square of the separation distance between the ions (1 / R2), and 'C' is proportionality constant. Coulomb's law mathematically describes the electrostatic forces that exist between stationary charged particles. These electrostatic forces give rise to some of the strongest bonds in nature, and it is precisely this type of bonding that gives ionic solids and salts their many unique configurations and properties.
Enjoy the Atoms!